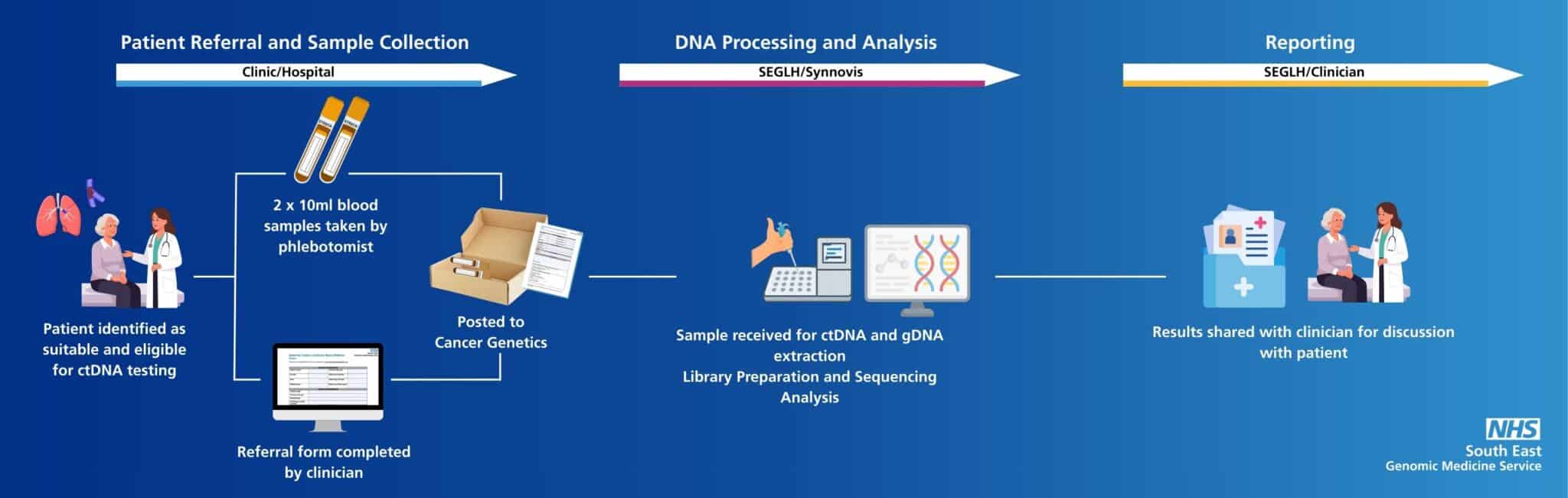
ctDNA testing
Circulating tumour DNA testing is now available to order by South East clinicians

Circulating tumour DNA testing is now available to order by South East clinicians..
The South East Genomic Medicine Service is the third centre to provide ctDNA testing for the NHS which is currently available for non-small cell lung cancer and breast cancer. This ‘revolutionary’ blood test was made freely available to all patients that meet the clinical eligibility criteria in 2025.
This page will support clinicians to understand the new pathway and answer your questions.
What is ctDNA testing?
The NHS is the first health service in the world to roll-out a ‘blood test-first’ approach to diagnosing suspected lung cancer, with the test being used before traditional tissue biopsies. As a result, patients are able to start treatment for lung cancer up to two weeks earlier.
Patients with advanced breast cancer whose cancer has not responded to previous treatment can also have a ctDNA test to determine if they are eligible for targeted treatments.
The blood test, also called a ‘liquid biopsy’, can detect tiny fragments of circulating tumour DNA (ctDNA) and looks for specific genetic variations of cancer, allowing patients to access targeted therapies which are tailored to the genetic profile of their cancer.
This service is centrally funded for all eligible patients with the three centres providing ctDNA testing across all of England. All referrers from the South East of England, which covers South London, Surrey, Sussex, Kent & Medway, should send sample to the South East Genomic Medicine Service for ctDNA testing.
Testing details
The South East Genomic Medicine Service will use the MSK-ACCESS® panel powered with SOPHiA DDM™
This NGS panel has been fully validated and can detect small nucleotide variants in 147 genes, including the TERT promoter region and MET exon 14 skipping, copy
number variants in 71 genes and 10 gene fusions detected by DNA based testing.
Find further information here.
Reporting and full interpretation on the front page of the report will be in line with the requirements of the National Genomic Test Directory for Cancer with inclusion of a list
of additionally detected oncogenic/likely oncogenic variants on page 2.
If full interpretation of any additional variants is required, a referral to our solid tumour GTAB can be made by completing the GTAB referral form and submitting it by email to gst-tr.gtabsoutheastglh@nhs.net
Order blood collection kits
The blood collection kits are no longer funded centrally by NHSE; kits must be procured and funded by individual referring Trusts.
- Ordering kits
New blood collection kits with postage labels for Guy’s Hospital are now available to purchase from Alpha Laboratories Ltd.
The order number is VBC-KIT-GH-01
Please contact Alpha labs directly for details of pricing solutions@alphalabs.co.uk
- Using up existing stock
If you have existing stock of Streck tubes with the old Royal Marsden address labels, please contact Alpha Labs at solutions@alphalabs.co.uk stating the number of kits remaining, and they will provide updated address labels as a PDF at no additional cost. Please print these new labels and affix over the existing Royal Marsden shipping labels.
- Ordering stock for samples originating from GSTT or other Trusts with internal transport links e.g. KCH
Referrals from clinics with internal transport links do not need to procure the collection kits which include Royal Mail 24h labels.
Streck tubes can be ordered direct from Alpha labs, but they MUST be packaged carefully using the absorbent bay pouches as the tubes are glass and are prone to breakage if not appropriately protected for transport.
The available product codes for Streck tubes are:
- 218996 Cell-Free DNA BCT®, 6 x 10mL, CE
- 218997 Cell-Free DNA BCT®, 100 x 10mL, CE
- 230244 Cell-Free DNA BCT®, 1000 x 10mL, CE
And for absorbent bay pouches:
- AB015-100
- AB010-100
Please contact the Alpha Labs key account manager for pricing, sites in the southeast area are mainly covered by Eimear Malone on emalone@alphalabs.co.uk
- Collection kit and general queries
Please contact the Cancer Genetics laboratory via seglhsomaticcancer@synnovis.co.uk if you have any questions.
- Laboratory address
Samples should be sent to:
Cancer Genetics
Synnovis Genetics Laboratories
4th Floor Southwark Wing
Guy’s Hospital
SE1 9RT
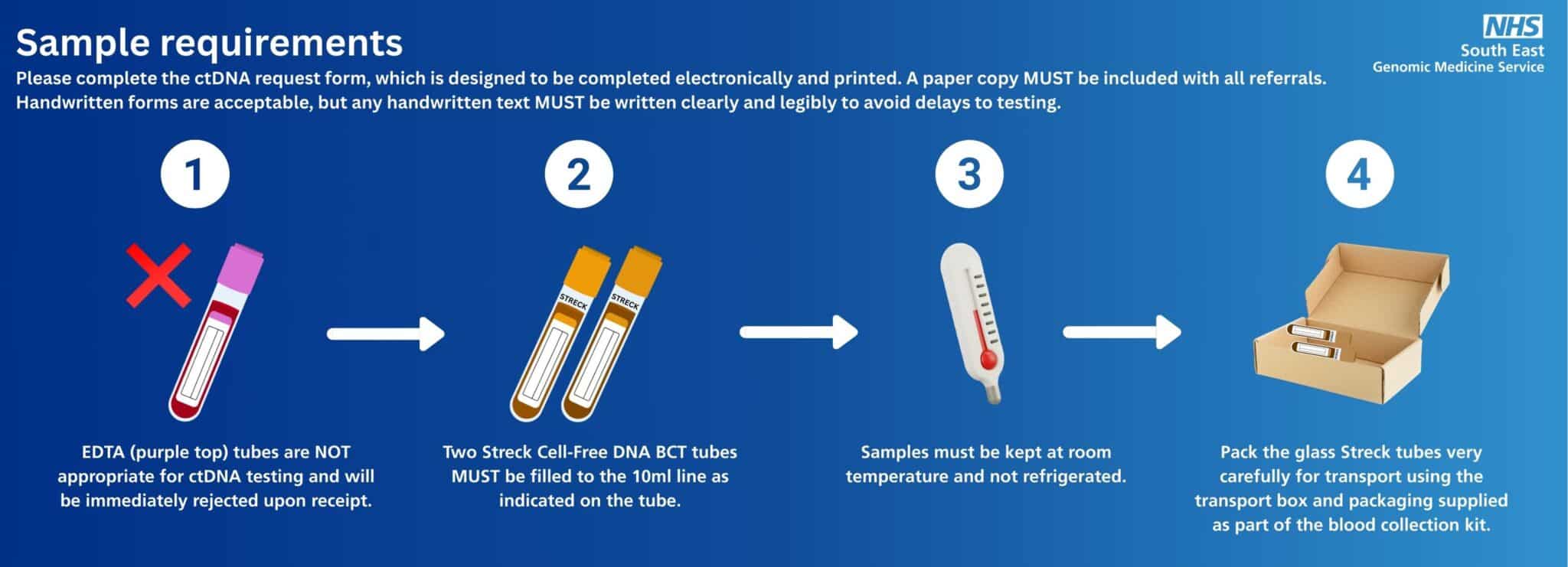
Sample requirements
Please complete the ctDNA request form, which is designed to be completed electronically and printed; a paper copy MUST be included with all referrals.
Handwritten forms are acceptable, but any handwritten text MUST be written clearly and legibly to avoid delays to testing.
Sample requirements for ctDNA are very different to other tests. Please follow these key steps:
-
Complete the ctDNA request form.
The form is designed to be completed electronically and printed; a paper copy MUST be included with all referrals.
Handwritten forms are acceptable, but any handwritten text MUST be written clearly and legibly to avoid delays to testing.
-
Take a blood sample.
Two Streck Cell-Free DNA BCT tubes MUST be filled to the 10ml line as indicated on the tube and mixed by gentle inversion 10 times.
Sample requirements for ctDNA are very different to other tests. EDTA (purple top) tubes are NOT appropriate for ctDNA testing and will be immediately rejected upon receipt.
-
Store samples at room temperature. They must not be refrigerated
-
Pack the glass Streck tubes very carefully for transport using the transport box and packaging supplied as part of the blood collection kit. The tubes are glass and prone to breakage if not packaged appropriately.
Post the sample to
Cancer Genetics
Synnovis Genetics Laboratories
4th Floor Southwark Wing
Guy’s Hospital
SE1 9RT
How do I find out more?
We hosted two education sessions to help clinicians understand the new process.
If you couldn’t join our live webinars you can access a recording here.
If you still have questions after watching, please do email seglh-ods@synnovis.co.uk