MT-RNR1 testing to guide aminoglycoside use
Clinicians working in multiple clinical specialties can request MT-RNR1 testing directly. There is no need to refer patients to Clinical Genetics for testing.
If you feel confident requesting MT-RNR1 gene testing, click the button below to access the necessary documents.
If you need more help arranging testing for your patient, the resources below should help.
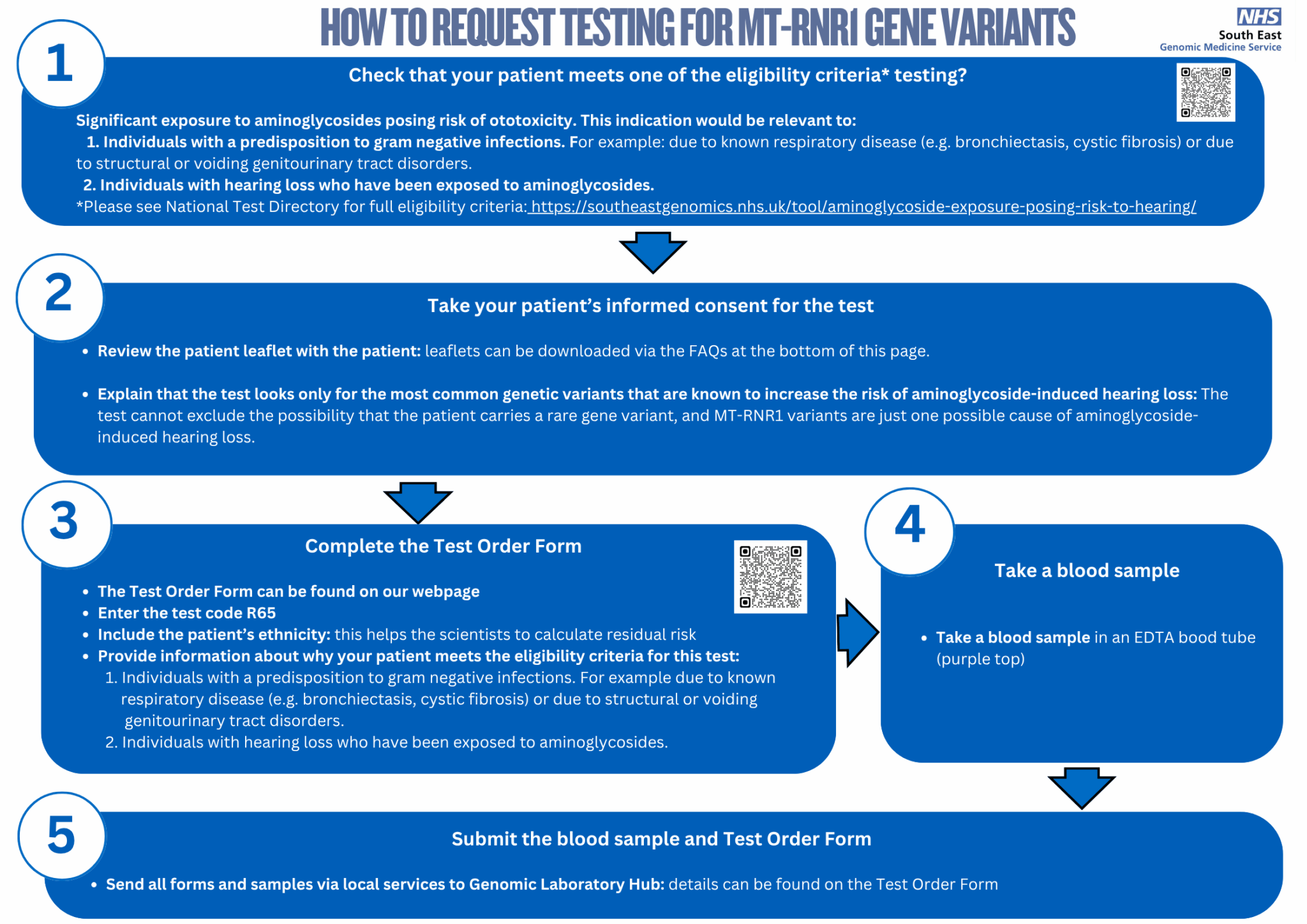
Follow the steps in this diagram to request MTRNR1 testing for your patient.
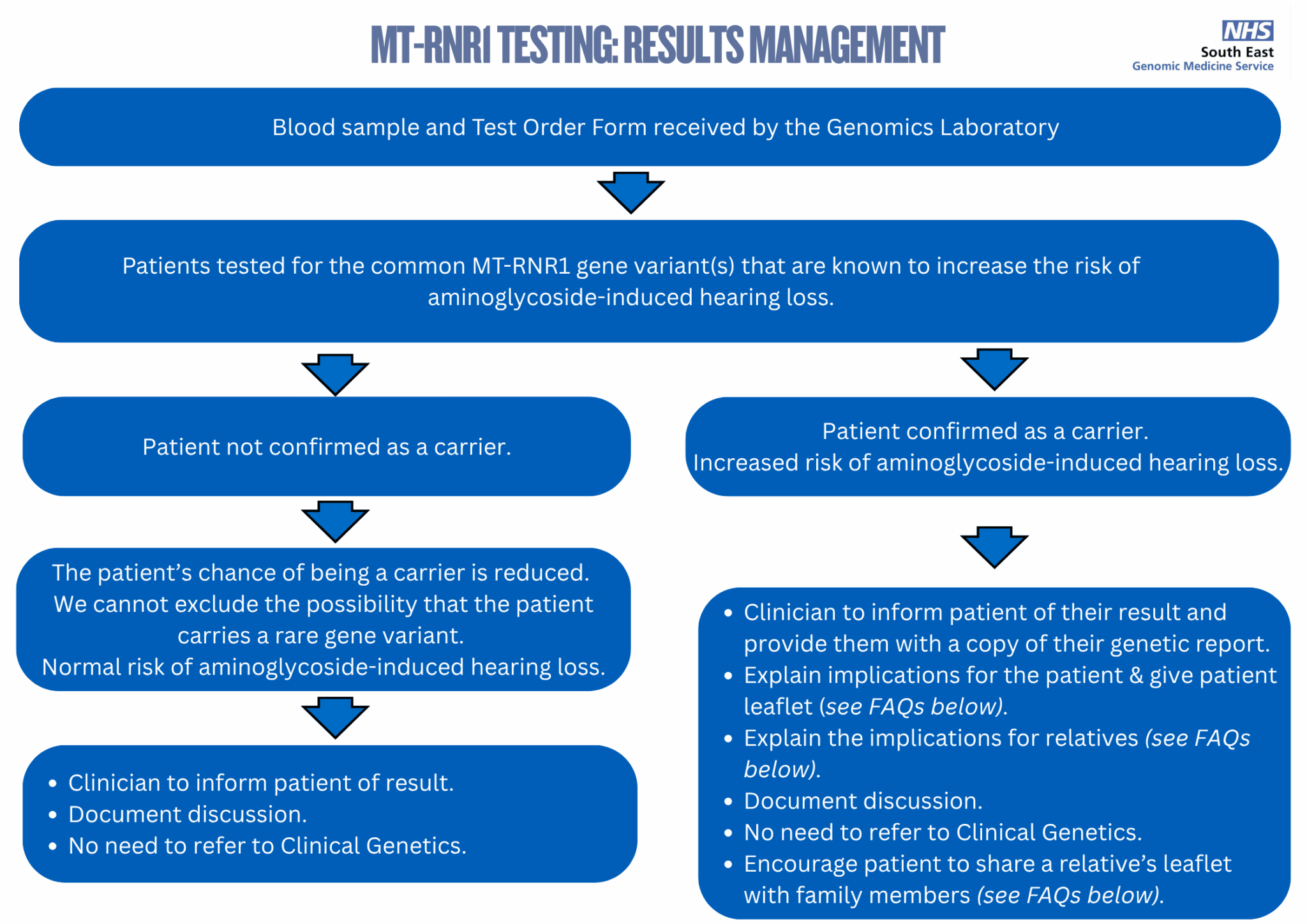
Follow the steps in this diagram to check what you need to do once you receive your patient's genetic report.
GeNotes: Knowledge Hub
Extend your learning with this encyclopaedia of resources, designed to support your understanding of genomic testing to guide aminoglycoside use
GeNotes In the Clinic Resource: Arranging Testing
Focused on the point of patient care, this short scenario looks at how to arrange genomic testing for a patient who is likely require broad-spectrum antibiotics during his disease course
GeNotes In the Clinic Resource: Dealing with a Positive Result
Focused on the point of patient care, this short scenario looks at what to do when presented with a patient who has a genetic variant predisposing her to aminoglycoside-induced hearing loss.
Frequently asked questions
There is an increased risk of aminoglycoside-associated ototoxicity including aminoglycoside-induced hearing loss (AIHL) in patients who carry variants in the mitochondrial MT-RNR1 gene, even if aminoglycoside serum levels are within the recommended range during treatment.
MT-RNR1 testing is a pharmacogenetic test to identify variants that can cause AIHL, so that alternative treatment options can be considered.
GeNotes has produced a helpful summary about aminoglycoside antibiotics and genetic variants in the MT-RNR1 gene: GeNotes Aminoglycoside Antibiotics
The Medicines and Healthcare products Regulatory Agency (MHRA) has published a Drug Safety Alert that includes advice to healthcare professions regarding aminoglycosides and increased risk of deafness in patients with mitochondrial mutations: MHRA Drug Safety Alert Aminoglycosides and increased risk of deafness in patients with mitochondrial mutations
Patients can be offered MT-RNR1 testing via the South East Genomic Medicine Service (Test code: R65) if they meet any of the following criteria:
- Individuals with a predisposition to gram negative infections.
- For example, due to known respiratory disease (e.g. bronchiectasis, cystic fibrosis) or due to structural or voiding genitourinary tract disorders.
- Individuals with hearing loss who have been exposed to aminoglycosides.
Clinicians working in multiple clinical specialties can request MT-RNR1 testing directly.
Referrals for testing will be triaged by the Genomic Laboratory; testing should be targeted at those where a genetic or genomic diagnosis will guide management for the proband or family.
Note that the associated test “R67 Monogenic hearing loss” should be requested for individuals with unexplained hearing loss.
m.1555A>G is by far the most common MT-RNR1 variant, and therefore the most clinically relevant, with a population prevalence of around 1 in 500 in the UK.
However, the prevalence may be higher in some ancestral groups for example the prevalence of m.1555A>G in individuals of East Asian ancestry is around 1 in 50.
The R65 laboratory test looks for the three MT-RNR1 variants known to cause an increased risk of AIHL; m.1555A>G, m.1494C>T, and m.1095T>C.
This means that whilst a “normal” or “no variants detected” result is reassuring, the test cannot exclude the possibility that someone carries a rare mitochondrial variant that would not be detected by the test.
Variants in the MT-RNR1 gene are only one possible cause of increased risk of AIHL. Individuals with no detectable variant should therefore still be considered as having a normal risk of AIHL.
Funding for genetic investigations is now held by the genomic medicine service laboratory hub. Appropriate specialist referring clinicians in secondary care can request this genetic investigation directly for eligible patients and the referring Trust will not be charged.
Before arranging testing, you should explain the possible outcomes of the test to your patient.
You then need to complete a Test Order Form and obtain a blood sample in an EDTA tube (purple top).
The Test Order Form and blood sample should be sent to:
Genetics Laboratory
5th Floor Tower Wing
Guy’s Hospital
London
SE1 9RT
You can download copies of the Test Order Form from the Aminoglycoside exposure posing risk to hearing testing page
The sample should be collected in an EDTA tube (purple top).
Sample Requirements: Each sample must be sent labelled with 3 patient identifiers and must state the sample type clearly on the sample container.
Sample Rejection: Samples may be rejected for the following reasons:
- Samples and request form do not show at least three identical patient identifiers
- The sample is in the incorrect collection media
- The request form is not sufficiently completed
- The sample is not of sufficient volume
- The sample is too old
Please include the reason for offering the patient a test. For example: pre-emptive testing for a patient with a predisposition to needing aminoglycoside treatment e.g. due to cystic fibrosis, or haemodialysis OR diagnostic testing for a patient with hearing loss and known exposure to aminoglycosides.
If the patient is having testing because their relative has been found to have a causative gene variant, please include details of the affected relative (name and DoB) and your patient’s relationship to the relative e.g. brother. It is also helpful to include some information about which area of the country the relative lives in; this will help the lab to track down the relative’s genetic report if required.
Note that the associated test “R67 Monogenic Hearing Loss” should be requested for individuals with unexplained hearing loss.
This test is a non-urgent standard test with an average turnaround time of 4-6 weeks.
All our turnaround times are listed on our specific turn around page: https://southeastgenomics.nhs.uk/professionals/service-turn-around-times/
Referrals for testing will be triaged by the Genomic Laboratory; testing should be targeted at those where a genetic or genomic diagnosis will guide management for the proband or family.
Genetic testing should not delay urgently needed aminoglycoside treatment but may be considered prior to needing aminoglycosides in patients with a pre-disposition to gram-negative infections especially in patients likely to require long-term or recurrent treatment with aminoglycosides.
The code for MT-RNR1 testing is “R65 Aminoglycoside exposure posing risk to hearing”. Please write this on the test request form.
Please write your name and contact details in this box. As the requesting clinician, you will receive the report and it is your responsibility to inform the patient of the result.
The laboratory is only able to email genetic test results to the email address that you provide on the form. It may be helpful to use a ‘group’ or departmental email address to reduce the chances of a result email being missed.
Carriers of MT-RNR1 variants that cause increased risk of AIHL should avoid aminoglycoside antibiotics (gentamicin, amikacin, neomycin and tobramycin).
Add an entry in the patient’s electronic health record and share the finding with the patient’s GP so that it can be recorded in their primary care electronic health record and their shared care record. See section: What information should be added to patient records? below
Patients with known MT-RNR1 variants OR a family history of MT-RNR1 variants OR a family history of ototoxicity with aminoglycosides are advised to inform their doctor or pharmacist before they take an aminoglycoside.
In addition to predisposing to AIHL, there is weak evidence that the m.1555A>G variant is also associated with non-aminoglycoside-related sensorineural hearing loss. Referral to audiology for a baseline hearing test should be considered according to local pathways and is recommended for any individual with a history of hearing loss or hearing changes.
Patients should be encouraged to share this information with family members so they can also avoid aminoglycosides and advise their doctor or pharmacist.
The Clinical Genetics service at St George’s University Hospitals NHS Foundation Trust has produced patient information leaflets for patients, and for relatives of patients who have been found to carry an MT-RNR1 variant. These can be found in the Is there a patient information leaflet? section below.
MT-RNR1 variants are subject to mitochondrial inheritance, so positive results are relevant to relatives along the maternal line. This means that MT-RNR1 variants are passed down from mother to child. MT-RNR1 variants are not inherited from the father. If an individual is found to carry an MT-RNR1 variant, their siblings, mother and relatives on their mother’s side of the family may be at risk and should follow the following advice:
- Maternal relatives of an individual who has been found to carry an MT-RNR1 variant should avoid aminoglycoside antibiotics.
- People with a family history of MT-RNR1 gene variants are advised to ask their GP to document in their medical record that they have a family history of an MT-RNR1 gene variant in a maternal relative and that they should avoid aminoglycoside antibiotics.
- People with a family history of MT-RNR1 gene variants are advised to inform their doctor or pharmacist before they take an aminoglycoside antibiotic.
In most instances, maternal relatives of a carrier of a variant in the MT-RNR1 gene do not need to be referred to Clinical Genetics for cascade testing. If they meet the eligibility criteria for R65 testing, their treating clinician can request MT-RNR1 testing directly. See section: Is my patient eligible for MT-RNR1 testing?
The Clinical Genetics service at St George’s University Hospitals NHS Foundation Trust has produced patient information leaflets for patients, and for relatives of patients who have been found to carry an MT-RNR1 variant. These can be found in the Is there a patient information leaflet? section below.
There is further information about Mitochondrial Inheritance in the GeNotes Knowledge Hub Mitochondrial inheritance — Knowledge Hub
An entry should be added against the patient’s electronic prescribing record if they are known to carry a mitochondrial variant associated with increased risk of AIHL:
Drug allergy section
Although not a true allergy, it may be helpful to add information on the patient’s test and result in the Drug Allergy section of their record. This is so that the information can be transferred with the patient across care settings.
For maternal relatives of a patient known to carry a mitochondrial variant associated with increased risk of AIHL an entry can also be added to the Drug Allergy section of their record as it is also recommended that they avoid aminoglycoside antibiotics.
SNOMED CT codes
For reporting in electronic records, the following SNOMED CT codes can be used to ensure consistent recording:
2365501000000102
- At increased risk of aminoglycoside-induced hearing loss due to mitochondrially encoded 12S ribosomal ribonucleic acid genotype (finding)
- At increased risk of aminoglycoside-induced hearing loss due to MT-RNR1 (mitochondrially encoded 12S ribosomal ribonucleic acid) genotype
2365511000000100
- At normal risk of aminoglycoside-induced hearing loss based on mitochondrially encoded 12S ribosomal ribonucleic acid genotype (finding)
- At normal risk of aminoglycoside-induced hearing loss based on MT-RNR1 (mitochondrially encoded 12S ribosomal ribonucleic acid) genotype
2365491000000108
- Mitochondrially encoded 12S ribosomal ribonucleic acid variant analysis (procedure)
- MT-RNR1 (mitochondrially encoded 12S ribosomal RNA) variant analysis
GeNotes has produced a helpful summary about aminoglycoside antibiotics and genetic variants in the MT-RNR1 gene: GeNotes Aminoglycoside Antibiotics
The Cystic Fibrosis Trust has produced a patient leaflet explaining MT-RNR1 testing: Cystic Fibrosis Trust Patient Information Leaflet Testing for potential increased risk of hearing loss with aminoglycoside antibiotics
The Clinical Genetics Team at St George’s Hospitals NHS Foundation Trusts have produced a patient leaflet for patients who have been found to carry a MT-RNR1 gene variant associated with increased risk of AIHL : Download Patient Information Leaflet
The Clinical Genetics Team at St George’s Hospitals NHS Foundation Trusts have produced a leaflet for relatives of patients who have been found to carry a MT-RNR1 gene variant associated with increased risk of AIHL : Download Relative Information Leaflet
The Royal National Institute for Deaf People (RNID) website provides information and support on ear health and types and causes of deafness including ototoxic drugs and hearing loss: www.rnid.org.uk .
The Cystic Fibrosis Trust has produced a patient leaflet explaining MT-RNR1 testing: Cystic Fibrosis Trust Patient Information Leaflet Testing for potential increased risk of hearing loss with aminoglycoside antibiotics
The Clinical Genetics Team at St George’s Hospitals NHS Foundation Trusts have produced a patient leaflet for patients who have been found to carry a MT-RNR1 gene variant associated with increased risk of AIHL : Download Patient Information Leaflet
The Clinical Genetics Team at St George’s Hospitals NHS Foundation Trusts have produced a leaflet for relatives of patients who have been found to carry a MT-RNR1 gene variant associated with increased risk of AIHL : Download Relative Information Leaflet
The Clinical Genetics Team at St George’s Hospitals NHS Foundation Trusts have produced a leaflet for relatives of patients who have been found to carry a MT-RNR1 gene variant associated with increased risk of AIHL : Download Relative Information Leaflet
If you cannot find the answer to your question on this page, you can contact your local Clinical Genetics service for support.
Clinical Genetics at Guy’s and St Thomas’s NHS Foundation Trust: gstt.geneticscorrespondence@nhs.net
Clinical Genetics at St George’s University Hospitals NHS Foundation Trust: genetic.secretaries@stgeorges.nhs.uk
Want to learn more about Genomics? Check out our resources for your specialty.