South East GLH
- About the Genomic Laboratory Hub
- Meet the team
- Order a test
- National test directory
- Getting the results
- 100k guidance for clinicians
The NHS South East Genomic Laboratory Hub
NHS England have reconfigured genetic laboratory services and commissioned 7 national Genomic Laboratory Hubs (GLH). The national GLH network will improve patient access for genetic testing and support the development of more personalised healthcare. The ultimate ambition is for patients with rare inherited diseases and cancer to be diagnosed and treated quicker than ever before, wherever they live.
The service allows clinicians to access testing for over 500 conditions, with some results being available in as little as three days. It will also enable the identification of gene mutations in cancer cells which can be targeted by new drug therapies.

The NHS South East Genomic Laboratory Hub (SE GLH), a network of leading foundation trusts and pathology providers, has been commissioned to deliver genomic testing services across South London, Kent, Surrey and Sussex. The SE GLH provides genetic and genomic testing for a total of 20 acute NHS Trusts and 9 non-acute NHS Trusts, ensuring equitable access to testing for a population of 8.4 million.
The SE GLH has also been commissioned to deliver specialist testing in 6 categories: cardiology, respiratory, gastro-hepatology, haematology, neurology and skin.
South East Consortium NHS Trusts






External Partners





Meet the GLH team
Sean Whittaker
Sean Whittaker
Deborah Ruddy
Deborah Ruddy
Richard Hall
Richard Hall
Deborah Morris-Rosendahl
Deborah Morris-Rosendahl
David McKinnon
David McKinnon
Deborah Yallop
Deborah Yallop
Nirupa Murugaesu
Nirupa Murugaesu
Debashis Sarker
Debashis Sarker
Felix Chua
Felix Chua
Kalnisha Naidoo
Kalnisha Naidoo
David Brawand
David Brawand
Nicholas Lea
Nicholas Lea
Gareth Gerrard
Gareth Gerrard
Michael Yau
Michael Yau
Nicola Husain
Nicola Husain
Heidy Brandon
Heidy Brandon
Nicholas Hickson
Nicholas Hickson
Gail Norbury
Gail Norbury
Jeremy Skinner
Jeremy Skinner
Olaf Hartberg
Olaf Hartberg
Rebecca Jebaratnam
Rebecca Jebaratnam
Richard Rider
Richard Rider
How to order a test
Genomic testing in the NHS is provided through a national testing network of seven Hubs. The South East Genomic Laboratory Hub coordinates genetic and genomic testing for the South London, Kent, Surrey and Sussex region. The tests will be aligned to the Cancer and Rare Disease National Genomic Test Directories. These directories specify which genomic tests are commissioned by the NHS in England, the technology by which they are available, the patients who are eligible to access the tests, and the healthcare professionals who can order them.
If you are not sure what test you want to order, take a look at the Genomic Medicine Service Test Selector, to help you find the correct test package for your patient https://test-selection-private.genomics.nhs.uk/test-selection/
Follow the links below to find out more and start the referral process:
I want to order a
Cancer test
I want to order a
Non-Cancer test
I want to order
Whole Genome Sequencing
Documents and forms
Shortcut to the GLH test request forms
National test directory
The National Genomic Test Directory
The 2022/2023 National Genomic Test Directory specifies which genomic tests are commissioned by the NHS in England, the technology by which they are available, and the patients who will be eligible to access to a test.
National Genomic Test Directory
Getting results
All genetic and genomic test results will be returned by the South East Genomic Laboratory Hub after appropriate interpretation and analysis has been completed. These reports will be returned directly to the ordering clinician, unless instructed otherwise.

Results will need to be discussed at a Genomic Tumour Advisory Board meeting prior to a report being issued. These meetings have been established to ensure there is appropriate expert knowledge and oversight of genomic test results and that they are managed and acted upon in a coordinated way. These GTAB meetings will take place on a regular basis across the South East region and responsible clinicians will be invited to their local meeting to review their patient’s results with the Board members. Below is a mapping of the overall MDT model through which genomic test results will be monitored and reviewed.
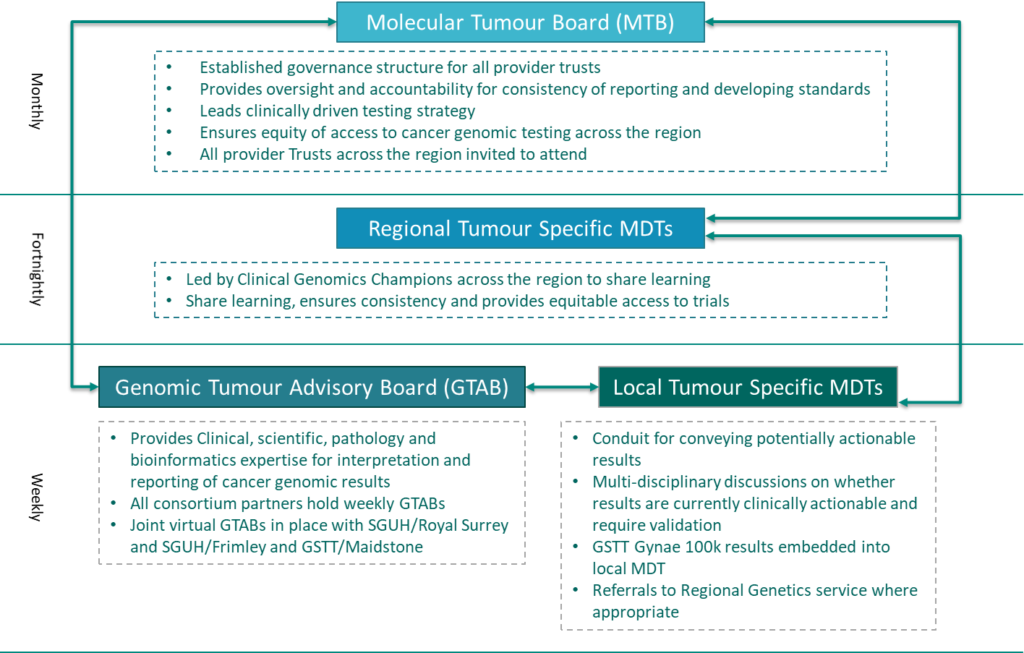

Results will need to be discussed by the Genomic Advisory team for Rare Disease at a MDT meeting prior to a report being issued. These meetings have been established to ensure there is appropriate expert knowledge and oversight of genomic test results and that they are managed and acted upon in a coordinated way. These GARD meetings will take place on a regular basis across the South East region and responsible clinicians will be invited to their local meeting to review their patient’s results. Below is a mapping of the overall MDT model through which genomic test results will be monitored and reviewed.

100k guidance for clinicians
A big thank you to all the healthcare professionals in the South London, Kent, Surrey and Sussex region who helped recruit over 2000 Cancer participants and nearly 4000 Rare Disease participants and their families to the Project. Recruitment to the Project has now come to an end and our current focus is on returning the results of the project to patients. Your continued cooperation and support are critical in ensuring this happens.
Referring clinicians may be invited to a Genomic MDT meeting to review the results and discuss next steps for the patient. Depending on this discussion, more work may be required before the final result can be returned to the referring clinician (i.e. further analysis and validation of the findings).
In some cases an MDT discussion may not be appropriate and the report may be issued directly after the analysis at the lab. The referring clinician is then responsible for discussing the result with the patient.
Information for healthcare professionals on the 100,000 Genomes Project can be found on the Genomics England website:
Further reading:
- Guide to support clinicians in returning results for patients with a suspected rare disease – NHS Genomics Education Programme
- Genomics England Rare Disease Results Guide
- The National Genomics Research and Healthcare Knowledgebase (previously the 100,000 Genomes Project Protocol)
- Example whole genome analysis (WGA) from the Cancer programme