Genomics Laboratory Services and COVID-19 Business Continuity Plan

Re: Genomics Laboratory Services and COVID-19 Business Continuity Plan
Dear Service User
I am writing to inform you of our current situation concerning the mobilisation of the South East Genomics Laboratory Hub operating model.
As you are aware the NHS is dealing with unprecedented pressures and the GLH Network is working with NHS England to mobilise services to support the national response to the COVID-19 outbreak.
This will have an impact on the provision of genomic testing and as directed by NHSE we have been working with clinical colleagues to implement measures to safeguard the most critical genomics tests.
As a result, roll out of some of the changes to deliver the South East GLH operating model have been postponed. Therefore, from 1st April you will not experience any changes to the way you order tests and your test provider and funding arrangement will not change unless new arrangements have already been finalised.
To ensure we continue to provide the most urgent genomics tests our business continuity plan is as follows:
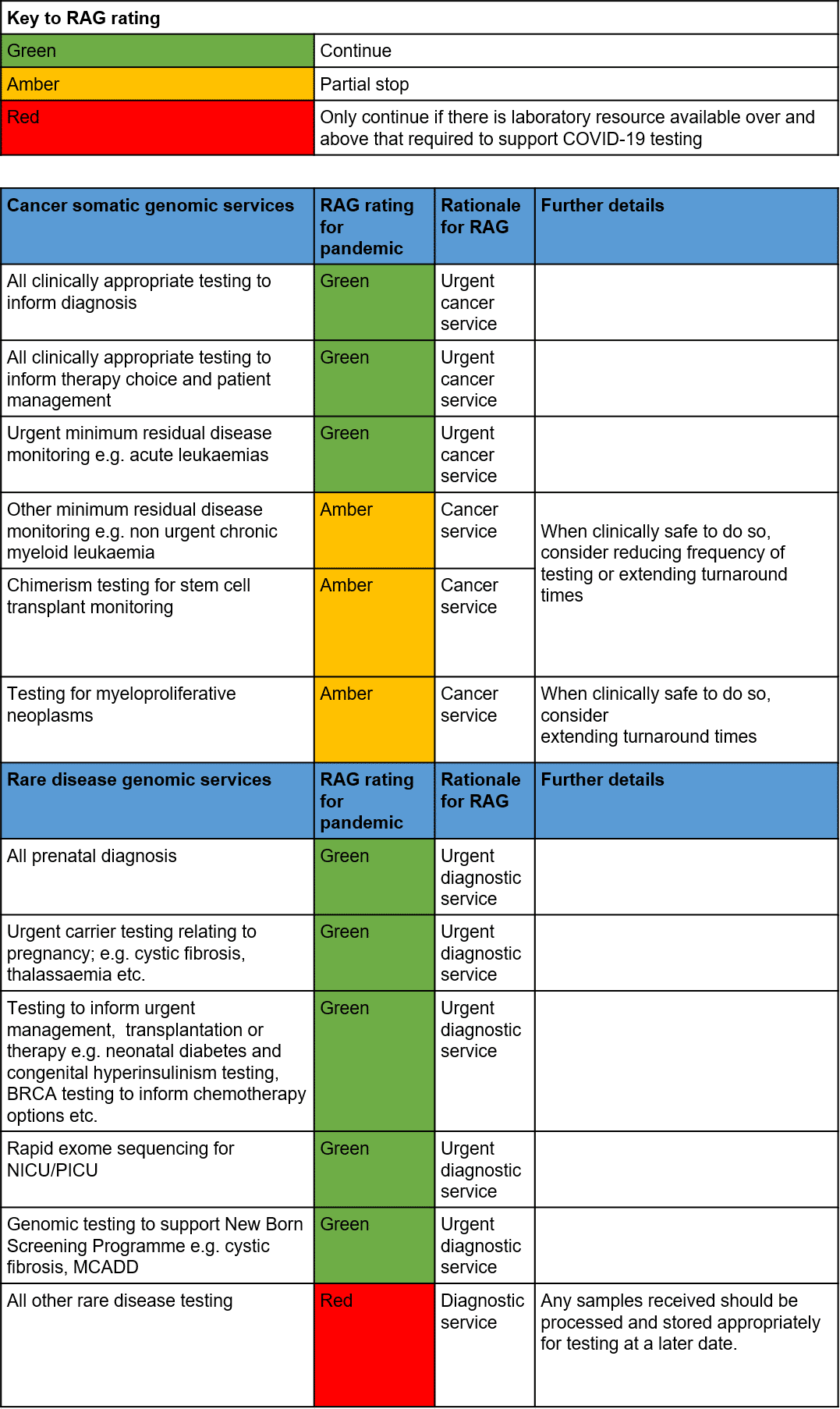
- Prioritisation of services has been agreed across the GLH Network with the support of NHS England. The list of tests can be found in Annex 1: National Business Continuity Plan (below).
- Prenatal and cancer testing will be prioritised and we aim to comply with the nationally agreed turnaround times. However, please be aware some delays may be experienced.
- During this period, please refrain from sending any non-urgent genomic tests. Any tests already sent to the laboratory will be processed but results will likely be delayed.
- We are continuing to assess our laboratories capacity on a daily basis to identify whether Amber or Red category tests can be delivered.
- As the number of staff that are required to self-isolate or redeploy increases, we have prepared plans to operate with minimum on-site staff at our laboratories in order to allow continuation of Green category testing.
We are aware that some clinics have implemented remote consultations by teleconferencing and are considering issuing DIY sample collection kits to patients. Please be aware we are unable to accept saliva/buccal samples as an alternative to blood for DNA extraction at this time.
This is a rapidly changing situation and we will continue to provide regular updates through our website. Where more detail is available, our local genomics laboratories will continue to contact you directly to inform you of the interim changes to their services. Mobilisation of the national Genomics Medicine Service and National Genomics Test Directory will resume once the situation has stabilised and we look for to working with you to launch the new services.
We appreciate your understanding and patience during this challenging time. If you have any questions or concerns please do not hesitate to contact us.
Many thanks and best wishes,
Richard Hall
SEGLH Operational Director
Annex 1: National Business Continuity Plan