A different kind of PPE #FoG2021
There were many important and thought-provoking topics discussed at this year’s Festival of Genomics and Biodata but one of the areas we found most interesting was the focus on Patient and Public Involvement (PPI) and Patient and Public Engagement (PPE).
But why do we need patient input?
Patient and public involvement and engagement is an important part of health-related research in the UK and is a statutory requirement under the Health and Social Care Act (2012). It is an opportunity for healthcare professionals to discuss their preliminary ideas with patients and members of the public as well as giving patients the opportunity to have input into shaping the project moving forwards.
Why we need to do it right
We need to think about the wider impact of genomic research at a community level and for different population groups, this is especially important for communities who are genetically distinct or identifiable. It is only possible to understand what potential harms to these communities may look like by conducting meaningful engagement in a local sensitive way.
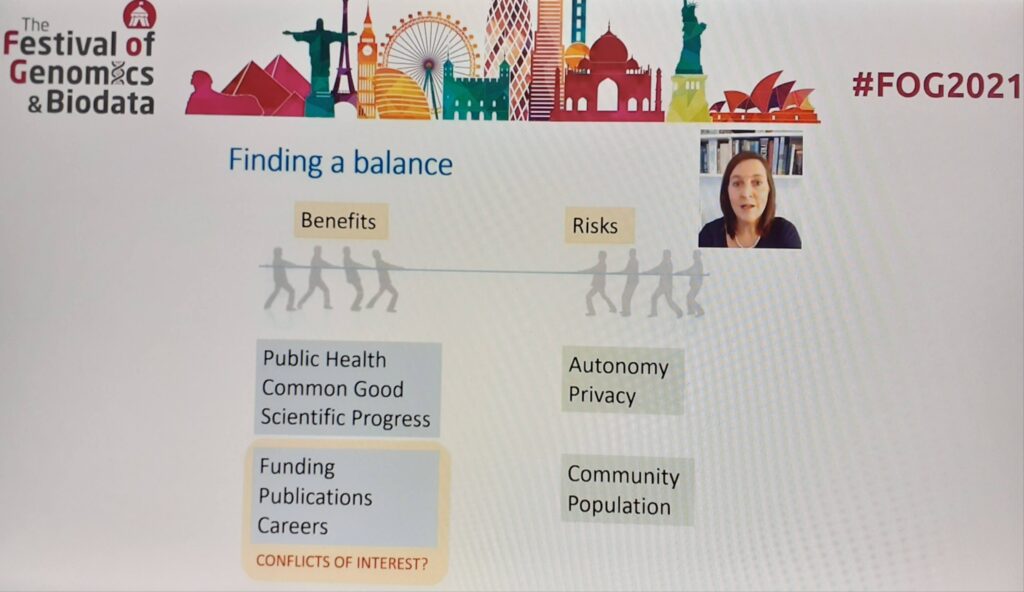
As we think about how our research can affect patients and communities, we must ask ourselves one important question: is science working on questions it finds interesting or targeting what’s important to patients? Having patients and the public involved at every step will remind us of this and the importance of the work being carried out.
Patients striking out on their own
Patients can be many things other than a patient, they can be a captain of industry in their own right, but being diagnosed with a rare disease is sometimes all we can see and they are brought down to the lowest common denominator. Finding a lack of support for people like themselves or effective treatments due to the disinterest of pharmaceutical companies has led many patients to form their own groups, representing their own interests.
A lot of the work of patient groups has been about educating doctors and looking at all the impacts of a disease on patients, using that knowledge to help improve their quality of life.
Alkaptonuria: a case study
Nick Sireau, Chair and CEO of the AKU Society, spoke about his nearly 20 year journey to get a treatment approved and available for alkaptonuria (AKU) patients around the world following his sons’ diagnoses. Alkaptonuira is an ultr-rare disease which, until a few months ago, had no approved treatment.
The AKU society developed a very strong relationship with the University of Liverpool and funded their own natural history study to better understand the disease. They were able to set-up the National AKU Centre at the Royal Liverpool University Hospital using a grant from NHS England after being able to demonstrate that it could save the NHS £82,000 per year per patient. Finally they set-up a 13-member EU-wide consortium which ran clinical trials for a potential drug, Nitisinone, and successfully worked to have it approved by the European Medicines Agency and the European Commission.
Following the successes of the AKU Society Nick co-founded Findacure, a UK charity building the rare disease community and upskilling patient groups. Please visit the AKU Society and Findacure websites for more information.
Bringing everyone together
When patients with the same condition come together they notice things in common that researchers haven’t and it becomes part of the phenotype. This only happens by bringing large groups of patients together and it is patient groups that can make that possible. While they often lack resources, one of the key benefits of patient groups is that they have very large networks of patients to tap into.
Patients have the capacity to bring different staff groups together, they work as convenors and align the different groups together for a single purpose. By involving patients and patient groups we can harness their resources and first-hand knowledge of their conditions and use it to drive meaningful and successful research.
Please visit our ‘how to get involved‘ page to find out how the SE GLH is implementing PPI and what you can do to take part.
